Table of Contents
On June 14, 2021, Philips announced a recall on several of its mechanical ventilators, Bilevel Positive Airway Pressure (BiPAP), and Continuous Positive Airway Pressure (CPAP) machines. The models affected were manufactured between 2009 and April 26, 2021.
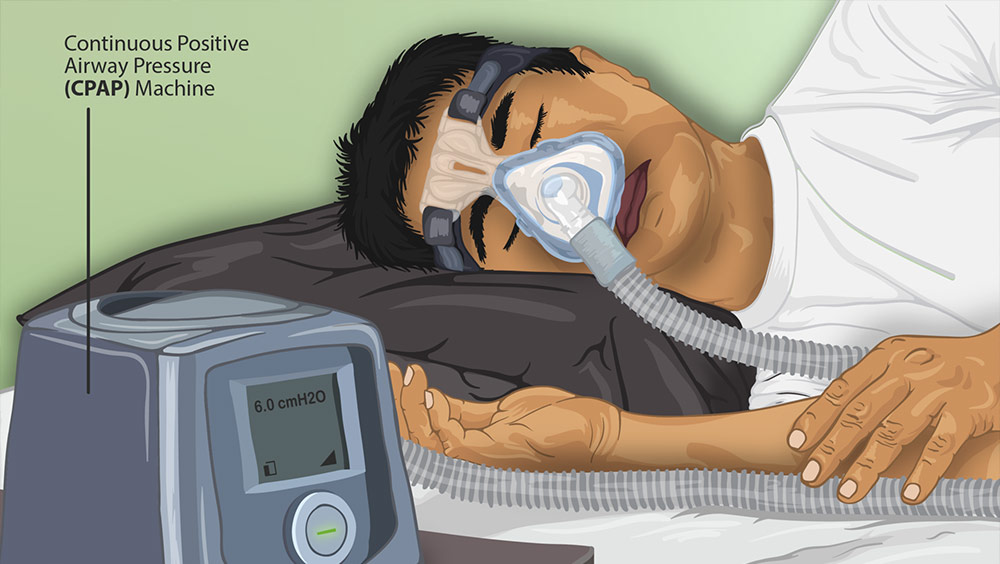
Philips CPAP machines help users get air into their lungs. These devices are commonly prescribed to people with forms of sleep apnea, like obstructive sleep apnea, a condition that causes abnormal breathing patterns while sleeping. Philips CPAP and BiPAP machines help pump air into the lungs while the person sleeps to support a more regular breathing pattern. For those with sleep apnea and other breathing conditions, these devices are necessities.
What Is a Medical Device Recall?
A medical device recall happens when a company recognizes a problem with one or more of its medical device models and takes steps to notify customers that the problem exists. The problem is something that violates guidelines set by the FDA, signaling that it could lead to potentially harmful side effects for users.
During a medical device recall, the FDA will monitor and work with the company issuing the recall to ensure that the company follows protocol. This could include everything from a company notifying users of the medical device to the company offering reimbursements, replacements, or repairs for the affected device.
Recalls do not always mean that a device must not be used anymore. Instead, some companies issue recalls when they conduct additional testing, inspection, or adjustments on the medical device. However, other recalls are more dire, resulting in the need for people to stop using their devices immediately and be monitored for health side effects.
Philips CPAP Recall: What We Know
Which Philips Models Are Being Recalled?
Several Philips CPAP, BiPAP, and mechanical ventilator models manufactured between 2009 and April 26, 2021, are involved in the recall notification. The table below includes the complete list of devices included in the Philips CPAP recall that pose a potential health risk.
CPAP and BIPAP Devices
| Device Type | Model (All Serial Numbers) |
| Continuous Ventilator, Minimum Ventilatory Support, Facility Use |
|
| Continuous Ventilator, Non-life Supporting |
|
| Noncontinuous Ventilator |
|
Ventilators
| Device Type | Model (All Serial Numbers) |
| Continuous Ventilator |
|
| Continuous Ventilator, Minimum Ventilatory Support, Facility Use |
|
| Continuous Ventilator, Non-life Supporting |
|
Why is Philips recalling CPAP and BIPAP machines?
Philips explains that the Philips CPAP recall on its breathing devices results from health risks associated with sound abatement foam, a type of polyester-based polyurethane (PE-PUR foam) that keeps the device’s vibration at a minimum while in use.
The Philips CPAP recall occurred when Philips became aware of issues with its sleep apnea machine models – specifically its mechanical ventilator device, BiPAP machine, and CPAP device. The affected devices experienced a breakdown of their sound abatement foam that could pose health risks for users. These sleep apnea devices may get particles or gasses from degraded foam that could make their way to the user of a Philips CPAP machine or another device.
This foam can degrade in high temperatures or high humidity. Philips also states that improper cleaning methods can lead to foam degradation, especially with ozone sanitizers and cleaners. The foam’s potential degradation on these machines can cause particles to seep into the machine, posing serious health risks to users.
While not all details have been released regarding why the foam might break down in each recalled device, Philips mentions that unapproved cleaning methods and the use of unapproved cleaners could be a potential cause. Philips has also indicated that high heat and humidity from environmental conditions could also cause foam degradation.
Since the announcement of the Philips CPAP recall, affected device owners have been left with many unanswered questions, including whether they can expect to receive a replacement and how necessary it is to stop using their machine immediately. The U.S. Food and Drug Administration (FDA) has been working with Philips to monitor its progress in remediating the situation and providing updated information to consumers.
Although Philips has voluntarily issued its recall, the FDA is closely monitoring the situation and the company to ensure that the proper steps are taken to remedy the problem. Some users are also preparing to file a Philips CPAP recall lawsuit as a result of lung injury or cancer thought to be caused by their CPAP machine.
What Are the Potential Side Effects of Philips CPAP Machines?
Most CPAP and BiPAP machines can cause a few side effects for users. Usually, these are cold-like symptoms that occur from minor irritation with pressurized air moving through the nose and mouth. Runny nose, congestion, nose bleeds, and dry mouth are commonly reported symptoms. Some users also experience headaches, eye irritations, and rashes around where the mask sits.
However, more serious complications may occur from using one of the recalled Philips CPAP, BiPAP, or ventilator machines. Although reports of these side effects have been minimal, Philips notes that the breakdown of its sound abatement foam can lead to:
- Asthma
- Cough
- Sinus pressure or infection
- Chest pressure
- Organ damage
- Headache
- Airway inflammation
- Respiratory tract irritation
Most importantly, the particles and gasses left behind from foam degradation can also cause carcinogenic effects.
Can I remove my device’s foam?
Because the primary issue of each machine included in the Philips CPAP recall is the breakdown of the sound abatement foam, you might be tempted to remove the foam to avoid potential health risks from foam particles or gasses. However, doing so can create additional risks and harm the machine.
Philips and the FDA recommend leaving the foam intact. Removing it could damage your machine or introduce new foam particles into the device’s air pathway.
How do I know if my Philips CPAP has been recalled?
If you know your Philips model name and number, you can use the list of products mentioned in the Philips CPAP recall to determine if your model is part of the recall.
If you’re not sure of your exact Philips model and whether it’s involved in the Philips CPAP recall, you can look up its serial number on the Philips website. Philips will let you know if your device is included and will walk you through registering your device to get notifications about the recall. You can also register your device on the Philips website to learn whether your sleep machine has been recalled.
Is there a recall on Philips DreamStation?
The Philips DreamStation lineup includes both CPAP and BiPAP machines. Some Philips DreamStation devices are named in the recall, including DreamStation ASV, DreamStation Go, and DreamStation ST, AVAPS. For a full list of recalled ventilator devices, you can check the table above.
Philips also allows you to look up your medical device serial number on its website to determine whether you have an affected PAP device.
Is there a recall on Philips Respironics?
We’ve seen some confusion regarding the details of the Philips CPAP recall. Philips Respironics is the brand name of Philips medical devices, like Philips ventilator machines and Philips sleep apnea machines. This brand name distinguishes Philips medical devices brand from its other brands.
The Philips CPAP recall is for medical devices under the Philips Respironics brand name. However, not all of the brand’s devices are named as recalled Philips CPAP machines. Refer to the table above for a full list of recalled devices.
Is Philips monitoring any other devices?
Currently, Philips believes that all affected devices have been accurately named in the recall based on the type of foam used, their manufacturing dates, and the model designs. However, there is always the potential for other devices to exhibit problems.
Philips has committed to monitoring its devices and taking reports from users seriously. The company is currently monitoring any reports of potential health risks from users, indicating that other models may also be affected.
Consumers are urged to register their devices with Philips to be the first to know if any recall status changes with their device.
What should I do if my machine has been recalled?
First, you should speak with your doctor about whether you should continue using your device or not. Your physician will weigh the risks and benefits of continued use as it relates to your specific condition.
If you’ve already registered your device and it’s been named in the recall, Philips should have reached out to you by mail regarding the recall and the next steps. However, customers who have not yet registered their devices may do so on the Philips website.
Once you do, Philips can contact you about a possible replacement or repair of your CPAP or BiPAP device.
If I stop using my CPAP or BiPAP device, will my disability benefits be impacted?
No. Because your device is involved in a documented recall, stopping the use of your CPAP or BiPAP device will not impact your disability benefits. However, it’s important to consult with your doctor before stopping use to ensure that it’s in your best interest.
Is it too late to register my Philips CPAP device?
No. If you haven’t yet registered your apnea device, you can do so on the Philips website. Go here and click on the Begin Registration Process link to get started. You can also call 877-907-7508 to speak with a representative who can assist you.
What do I do with my recalled device if I get a replacement?
Philips and the FDA ask that you do not throw away or recycle your old CPAP device if you get a replacement through the Philips replacement program. Instead, contact Philips via its recall notification page to request instructions for discarding your device.
If you received your device through your healthcare provider, you may also contact them for information.
How should I clean my CPAP device?
Because certain types of cleaners can harm the sound abatement foam on Philips CPAP devices, Philips stresses the importance of cleaning the device according to its instructions. Foam degradation can appear more frequently in devices in which an ozone cleaning device or ultraviolet light cleaner was used. Follow the manufacturer’s instructions to clean your device properly.
What We Don’t Know
How serious are the health effects?
Since before the Philips CPAP recall was officially released, Philips has been keeping track of reports of health issues potentially related to the affected devices. To date, some of the side effects consumers have reported include headache, chest pressure, sinus infection, cough, and upper airway irritation.
Philips also says that skin and eye irritation, asthma, kidney and liver damage, inflammatory responses, and carcinogenic effects are potential side effects of exposure to broken-down foam. However, the company continues to monitor reports to learn more about possible symptoms.
Are there any other Philips models affected?
As of now, Philips has recalled all devices it believes to be affected. Each recalled machine is listed on the Philips website, and you can also find the list in this article’s table. As Philips continues to monitor reports about its devices, the company will update its list, if necessary, with other devices.
How will the FDA monitor Philips during this recall?
The FDA has been closely monitoring Philips to ensure that the proper steps have been taken to recall the appropriate devices, notify consumers, and repair or replace affected devices. In addition to providing in-depth information about the recall to consumers, the FDA has conducted on-site inspections of Philips manufacturing facilities to monitor compliance.
The FDA is also monitoring the Philips plan to make sure that consumers with recalled machines get a repair or replacement as quickly as possible. However, the FDA currently does not have a set timeline for this to happen, but the agency is working with Philips to speed up the process as much as possible.
Should I stop using my device?
This answer will vary with each consumer. Users of recalled devices should consult with their doctor to determine whether stopping the use of their device is the best option. You and your doctor can review the risks associated with the continued use of your device until a replacement is available with the risks of stopping the use of the device as it relates to your sleep apnea or other condition.
Will Philips repair or replace devices?
Although the details have yet to be worked out, Philips has said that it will either repair recalled devices by replacing the sound abatement foam or replace devices that cannot be repaired. Philips is currently unaware of how long this program will take to implement or how long it might take to repair or replace devices. However, Philips announced its intentions to complete the programs within 12 months.
Can veterans receive a replacement device?
Yes. Anyone with a device named in the Philips CPAP recall may be eligible for either a repair or a replacement device, including veterans. However, it’s unknown how much time it may take to get your replacement device to you.
Will Medicare pay for new CPAP devices due to recall?
We are not yet sure how insurance companies like Medicare will handle the replacement of a sleep apnea machine from Philips. If it’s been a long time since your company has paid for a device, it may cover another one for you. However, if it’s been only a couple of years since you received your CPAP or BiPAP
machine, Medicare may not foot the bill for a replacement due to recall.
How long will it take to correct this issue?
Philips is not sure how long it will take to replace or repair all affected devices at this time. However, the company intends to complete its repair and replacement programs within 12 months.
What To Know About the Philips CPAP Recall
The Philips CPAP recall covers affected sleep apnea machine models, like CPAP and BiPAP devices. The recall stems from an issue with the sound abatement foam that insulates and quiets the machines during operation. Over time, due to improper cleaning or high heat and humidity, the foam may break down, leaving behind particles or emitting gasses that could be harmful to the health of device users.
The FDA is working closely with Philips to ensure that the company carries out the proper recall steps promptly. The FDA is monitoring Philips facilities and its repair and replacement programs designed to get affected devices replaced for consumers as quickly as possible.
If you aren’t sure whether your device is named in the Philips CPAP recall, you can check on the Philips website by registering your machine or by calling Philips at 877-907-7508 for assistance.